Articles from Magnus Medical, Inc.

Magnus Medical, Inc., a pioneering therapeutic neuromodulation company advancing precision medicine in brain health, today announced enrollment of the first patient at University of Massachusetts Chan Medical School (UMass Chan), a leading academic health system, in its U.S. Department of Defense (DoD)-funded, multi-center pivotal clinical trial designed to evaluate the safety and effectiveness of SAINT® neuromodulation therapy for the treatment of postpartum depression (PPD).
By Magnus Medical, Inc. · Via Business Wire · February 5, 2026

Magnus Medical, Inc., the company behind SAINT®, the first and only FDA-cleared, rapid-remission therapy for Major Depressive Disorder (MDD), today announced that the Centers for Medicare & Medicaid Services (CMS) has finalized the 2026 Hospital Outpatient Prospective Payment System (OPPS) rule, preserving the existing payment structure for its breakthrough SAINT neuromodulation therapy. This decision ensures that Medicare patients with MDD will continue to have access to SAINT therapy in hospital outpatient settings.
By Magnus Medical, Inc. · Via Business Wire · November 24, 2025

Magnus Medical, Inc., a pioneering therapeutic neuromodulation company transforming the treatment of neuropsychiatric disorders, today announced that the Centers for Medicare & Medicaid Services (CMS) has released the final hospital outpatient payment rates for its breakthrough SAINT® neuromodulation therapy. This important ruling significantly expands access to SAINT therapy for Medicare patients with treatment-resistant major depressive disorder (TR-MDD) in the hospital outpatient setting.
By Magnus Medical, Inc. · Via Business Wire · November 12, 2024

Magnus Medical, Inc., a pioneering therapeutic neuromodulation company transforming the treatment of neuropsychiatric disorders, today announced promising new clinical data on SAINT® therapy at the 2024 NYC Neuromodulation Conference, Aug 1-3. The research will be presented in two posters demonstrating the long-lasting benefits of SAINT treatment and its potential for personalized continuation therapy in people with treatment-resistant depression (TRD).
By Magnus Medical, Inc. · Via Business Wire · August 1, 2024

Magnus Medical, Inc., a pioneering therapeutic neuromodulation company transforming the treatment of neuropsychiatric disorders, today announced the commercial launch of the SAINT® neuromodulation system, a groundbreaking, rapid-acting therapy for treatment-resistant major depressive disorder (MDD).
By Magnus Medical, Inc. · Via Business Wire · April 30, 2024
Magnus Medical, Inc., a medical device company and developer of brain stimulation technology for the treatment of neuropsychiatric disorders, today announced that the American Medical Association (AMA) has issued new Category III Current Procedural Terminology (CPT) codes for targeted, accelerated intermittent theta burst stimulation (iTBS) for depression, encompassing Magnus’ SAINT™ targeted treatment. The new codes will be published January 1, 2024, and effective July 1, 2024.
By Magnus Medical, Inc. · Via Business Wire · October 23, 2023

Magnus Medical, Inc., a medical device company and developer of brain stimulation technology for the treatment of neuropsychiatric disorders, today announced that the first participants have been treated in the Open Label Optimization (OLO) Clinical Trial evaluating the effectiveness of the Magnus Neuromodulation System with SAINT™ Technology for the treatment of Major Depressive Disorder (MDD).
By Magnus Medical, Inc. · Via Business Wire · July 20, 2023

Magnus Medical, Inc., a medical device company and developer of brain stimulation technology for the treatment of neuropsychiatric disorders, today shared promising results from a clinical trial published in the Proceedings of the National Academy of Sciences indicating that treatment with SAINT™ Neuromodulation reverses abnormal brain signaling by changing the direction of brain signal flow in people with severe depression. Findings also reveal a new biomarker that may be helpful in the diagnosis and treatment of major depressive disorder (MDD).
By Magnus Medical, Inc. · Via Business Wire · May 15, 2023

Magnus Medical, Inc., a medical device company and developer of brain stimulation technology for treatment of neuropsychiatric disorders, today announced it received 510(k) clearance from the U.S. Food & Drug Administration (FDA) for the SAINTTM Neuromodulation System for the treatment of major depressive disorder (MDD) in adults who have failed to achieve satisfactory improvement from prior antidepressant medications in the current episode.
By Magnus Medical, Inc. · Via Business Wire · September 6, 2022
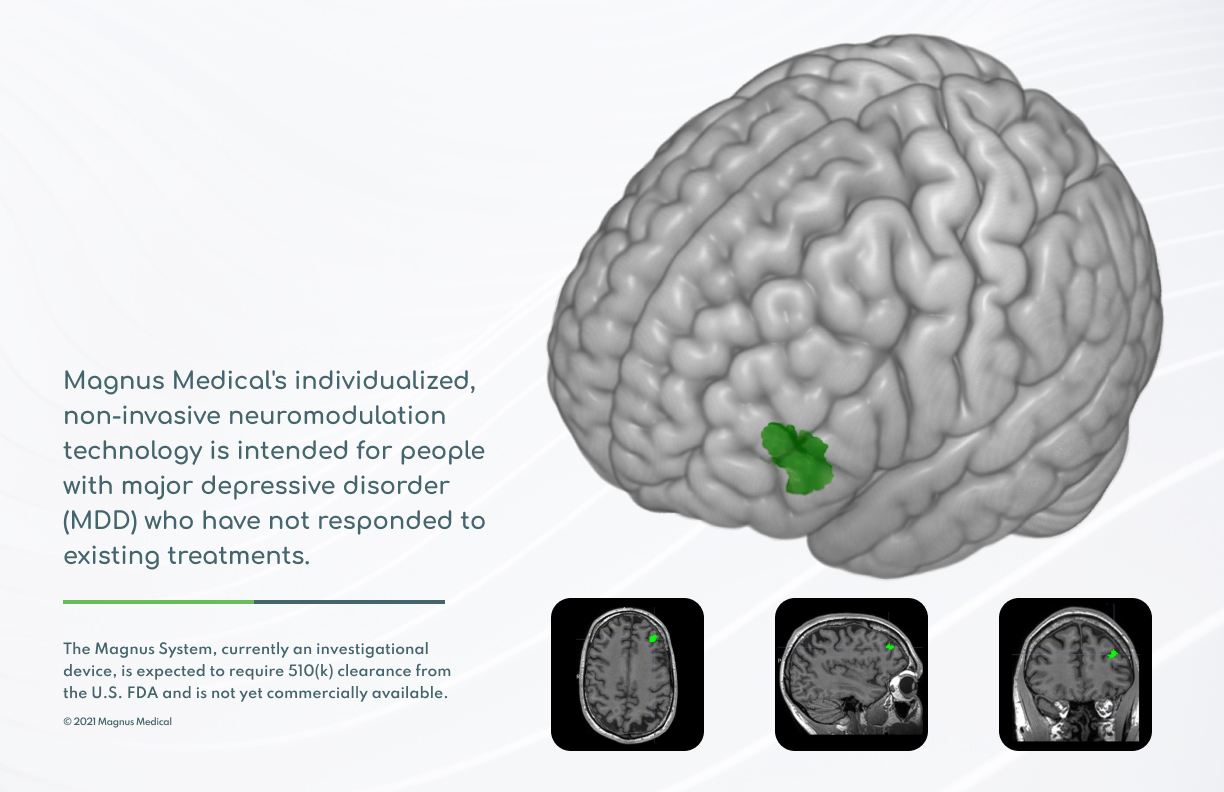
Magnus Medical, Inc., a medical device company, today announced that the U.S. Food & Drug Administration (FDA) has granted the company Breakthrough Device Designation for its individualized, rapid-acting, non-invasive neurostimulation technology designed to treat major depressive disorder (MDD) in people who have not improved sufficiently from antidepressant medication or other treatments. The FDA Breakthrough Device program is intended to help patients receive more timely access to breakthrough technologies that have the potential to provide more effective treatment or diagnosis for life-threatening or debilitating diseases or conditions than previous therapies.
By Magnus Medical, Inc. · Via Business Wire · October 29, 2021
